Abstract
Background. Anti-CD19 Chimeric Antigen Receptor (CAR) T-cells represent a major therapeutic advance in the management of patients (pts) with relapsed/refractory aggressive large B-cell lymphoma (R/R aggressive LBCL) with reported overall response rates between 40% and 83% in the pivotal trials (ZUMA1, JULIET, TRANSCEND) as well as in the real-life cohorts with either axicabtagene ciloleucel (axi-cel, Yescarta) or tisagenlecleucel (tisa-cel, Kymriah). However, a significant number of pts will experience failure after infusion, 20% to 35% of these failures occurring early during the first month. Lenalidomide is reported to activate CD8 T-cells, to inhibit regulatory T-cells and to restore T-cell immune synapse. Here, we report our experience with early exposure to Lenalidomide (LEN) in the treatment of early failure (<15 days) after axi-cel or tisa-cel for R/R aggressive LBCL.
Methods. Between June 2018 and June 2021, 142 patients with R/R aggressive LBCL were treated with commercial anti-CD19 CAR T-cells, axi-cel (n=70) or tisa-cel (n=72). With a median follow-up of 11.4 months [IQR, 4.4-20.4], 76 patients experienced a failure based on the Cheson 2014 response assessment criteria, including 36 (47%) within the first month. Among those 36 patients who progressed during the first month, LEN was initiated before D15 in 17 pts (EARLY LEN pts), and 19 were not treated by LEN (OTHER pts). Efficacy of early LEN was assessed by CT-scan and 18FDG-PET after the 1st cycle and at the end of treatment. CAR T-cells expansion was monitored in blood by flow cytometry. All pts gave informed consent.
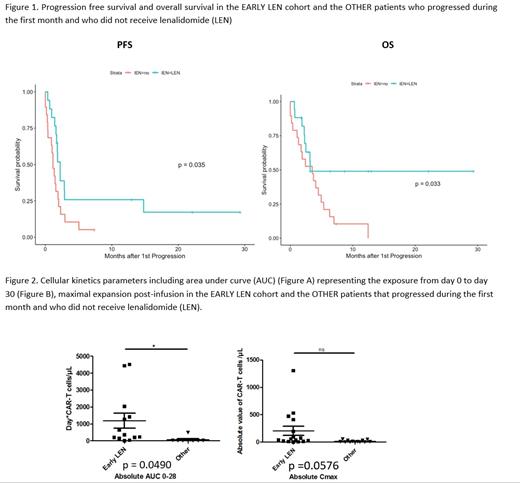
Results. Median age of the 17 EARLY LEN pts was 65 yo (IQR 59-71) and 7 (41%) male. Histology subtypes were 12 (71%) DLBCL, including 7 GCB and 5 non-GCB, 1 (6%) PMBL, 4 (24%) tFL; 10 (59%) were treated with at least 3 prior therapies including 2 high dose therapy plus autologous stem cell transplantation. All received bridging therapy with 16 (94%) of them receiving high intensity regimen with combined immunochemotherapy. At time of infusion, 14 (82%) presented with an advanced disease (stage III or IV), 14 (82%) an elevated LDH level, 7 (41%) had at least 2 extra-nodal sites; 3 (18%) with low age-adjusted International Prognostic Index (aaIPI), 2 (12%) with low-intermediate aaIPI, 6 (35%) with high-intermediate aaIPI, and 6 (35%) with high aaIPI. Median total metabolic volume (TMTV) was 144 mL [IQR, 109-45]). The best overall response rate was observed in 15 (88%) of the EARLY LEN patients, including best complete response in 6 and best partial response in 9. Compared to the OTHER pts, the median progression-free (PFS) was significantly improved, with a median PFS of 68 days (95% CI, 52- not reached) vs 35 days (95%CI, 28-70) (p=0.035). Among the 6 pts with LEN< D15 that obtained a CR, PFS was even better with 3 events at 20, 88 and 451 days. Median overall survival (OS) was 97 days (95% CI, 76-Not reached) in the EARLY LEN pts, compared to 107 days (95 CI, 53-192) in the pts who progressed during the first month (p = 0.033). In univariate analysis, the 13 EARLY LEN pts evaluable for expansion had a higher CAR T-cell expansion in blood during the first 28 days (mean AUC D0-D28=1184 days*CART Cells number/µL of total blood) than other pts relapsing (mean=66 , p=0.0490), including those treated with LEN after D15 (mean=313, p=0.0166). Otherwise, expansion of these 13 EARLY LEN pts was similar to expansion of patients without relapse (mean=737, p=0.214). Grade 3-4 neutropenia was observed in 13 (76%) pts, grade 3-4 thrombopenia in 11 (65%) and grade 3-4 anemia in 8 (47%). Cytopenias required LEN discontinuation for 2 pts (12%). Cytokine release syndrome occurred in 13 patients (76%) (10 axi-cel and 3 tisa-cel) with a median duration of 6 days, including 1 (6%) grade 3 and no grade 4. Immune effector cell-associated neurotoxicity syndrome occurred in 6 (35%) (2 axi-cel and 4 tisa-cel) with a median duration of 10 days, including 1 (6%) grade 3 and no grade 4.
Conclusion. Early LEN exposure at time of CAR T-cell expansion led to a high response rate in very high-risk pts of relapse after CAR T-cells. Comprehensive analyses of these mechanisms using tumor transcriptomic and single cell analyses should help for a comprehensive analysis on the peculiar mechanism of action of LEN, including not only an anti-tumoral effect, but also an immunomodulatory effect.
Di Blasi: Novartis: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal